
How Myoact got certified with FormlyAI
Physical therapy
Software
Class I
70k€Saved compared to alternative quotes
0Clinical trials needed
5Months time saved in document writing
The Challenge
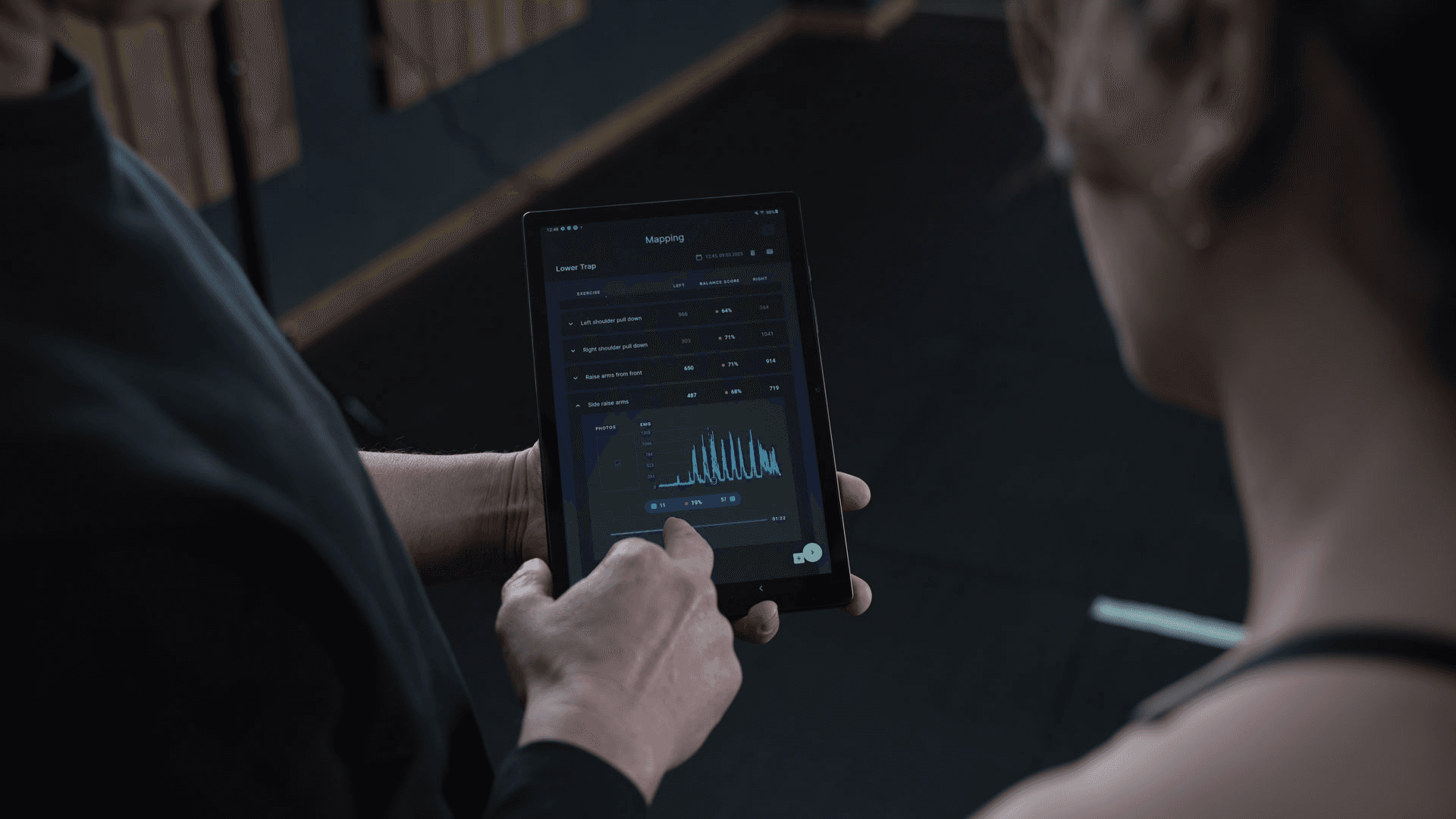
sEMG sensors measure the electrical activity that muscles produce during contraction, allowing users to identify muscle imbalances and understand their body in new ways, especially after injury recovery.Myoact developed a software application that connects to sEMG sensors, enabling users to view their muscle activity in real time, but recognized that certification was essential for market validation in a competitive landscape.The company approached Formly with the goal of achieving Class I certification under the EU's Medical Device Regulations, which would position them alongside other certified solutions and allow them to provide clinical analytical tools for healthcare professionals.Using Formly's eQMS and AI-powered documentation tools, Myoact quickly progressed from limited in-house documentation to full certification readiness, with training on QMS processes that enabled faster documentation updates for new software versions.Through strategic regulatory discussions with Formly, Myoact was able to certify their software as a standalone product without requiring dedicated hardware certification, and the achieved certification not only validated their product among competitors but also opened pathways to insurance reimbursement.
Our package with Formly is exactly what we wanted and what we needed. No BS, no fluff. Formly's services are straight to the point and the team is so pleasant to work with. As a small business and a startup, that's what you really need.
Anina LanghansCo-Founder & Product Lead LipoCheck
The Solution
Myoact successfully achieved their certification goals on time and within budget, demonstrating that small teams can navigate complex medical device certification processes when equipped with appropriate tools and support.The company now holds Class I certification for their software and continues to use Formly's eQMS to manage data and device surveillance, ensuring efficient handling of future compliance activities without disrupting software release cycles.Myoact is positioned for future growth with plans to pursue Class IIA certification as they expand their software's capabilities and functionality, confident that Formly's continued support will help them meet additional regulatory requirements efficiently.The team also plans to launch their product in the US market soon with Formly's assistance, allowing them to stay competitive in the rapidly evolving medical device landscape.

Level up your compliance
Get in TouchNo commitments required